



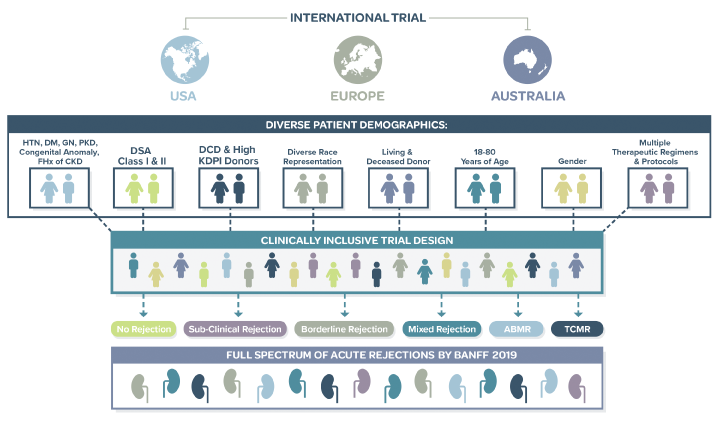
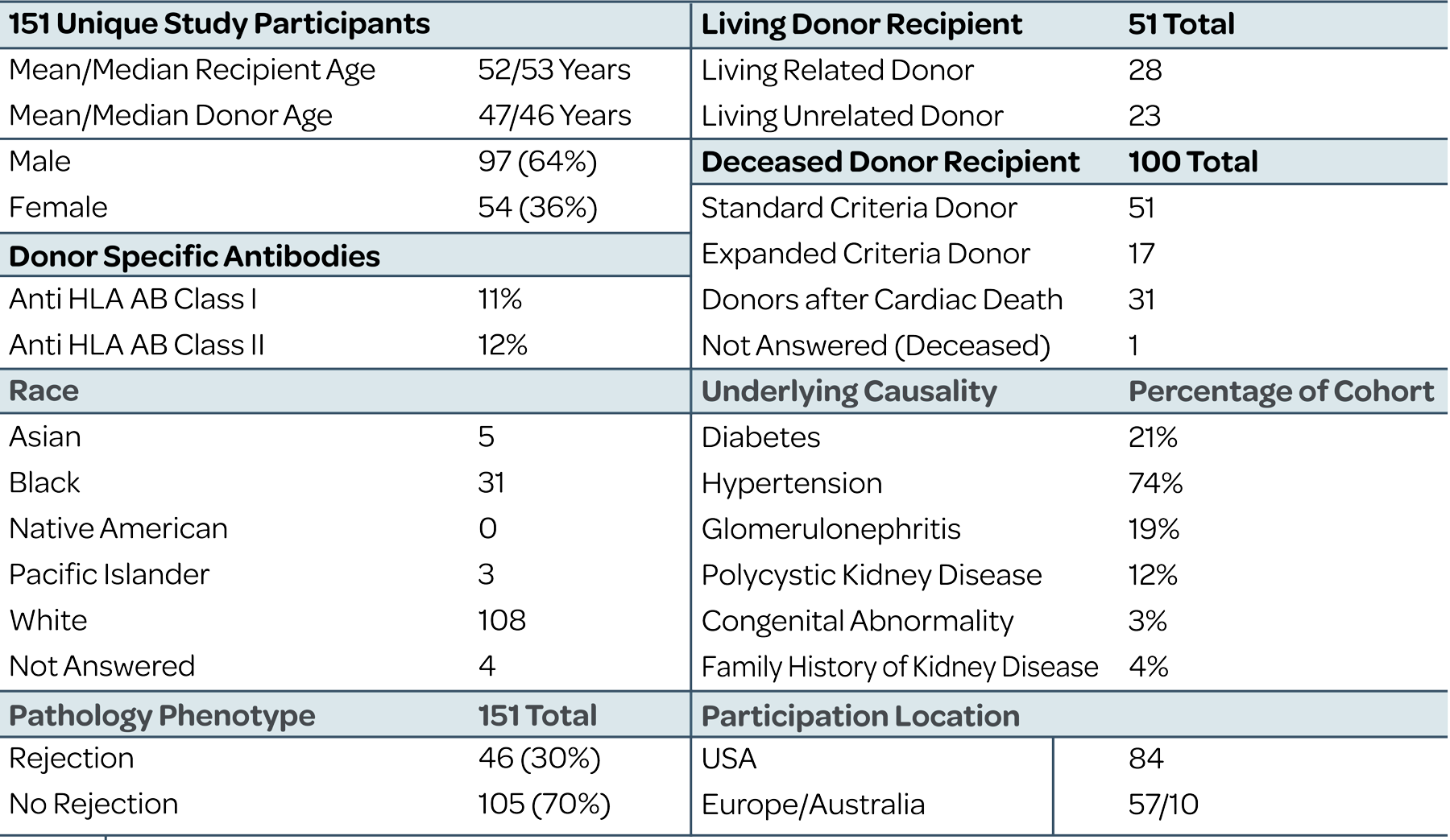

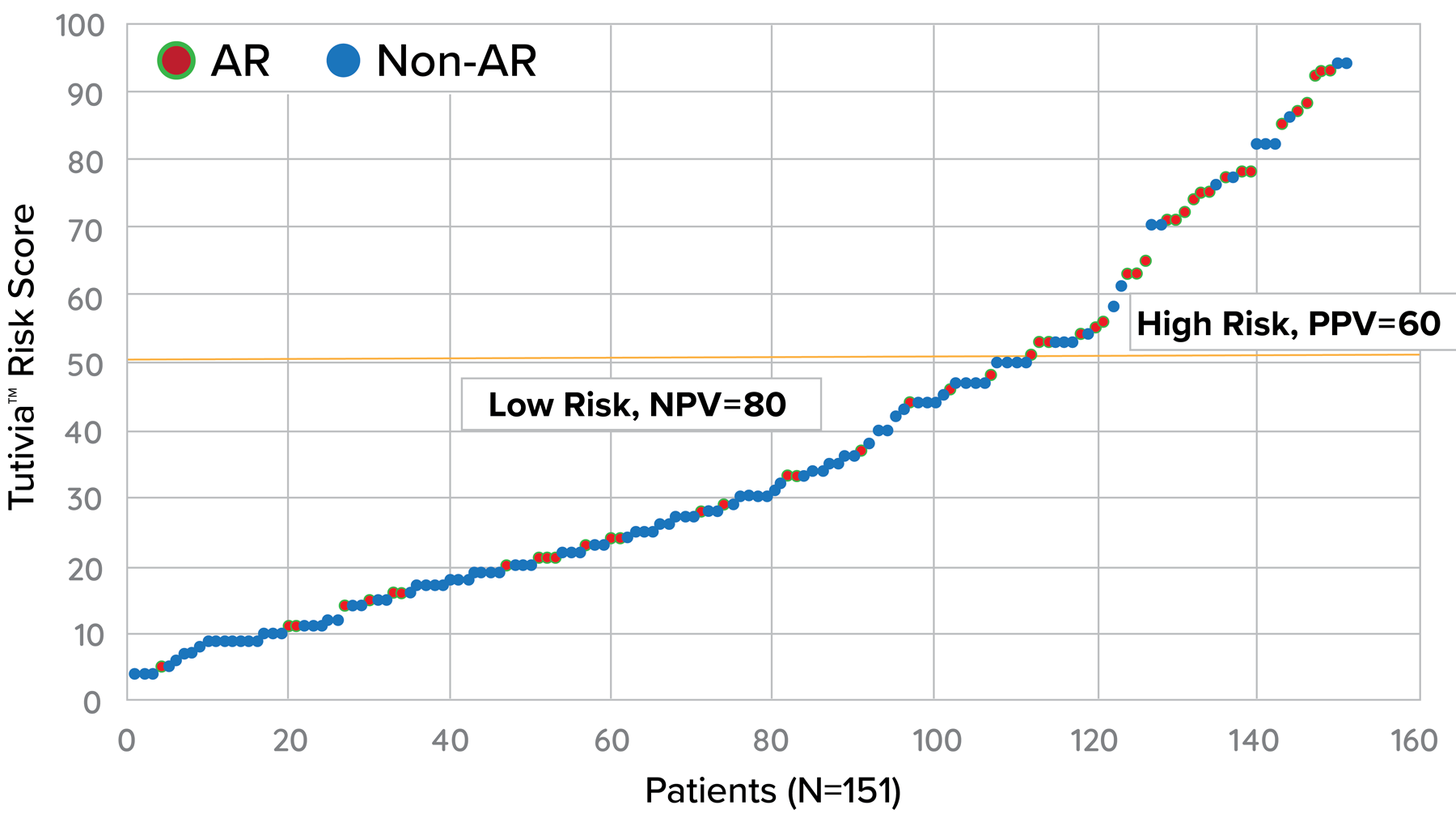
Tutivia™ risk scores are calculated continuously from 0-100; for interpretation, a pre-determined risk cut-point of 50 was established based on the independent training set1. The risk score distribution (fig. 1) resulted in 26.5% of patients with a high risk (>50) score result in which 60% were shown to correlate with rejection on histopathology; low risk score results in 73.5% of patients correlated with an absenceof rejection on histopathology findings in 80% of patients. The receiver operating characteristic curve AUC was 0.693, P<0.001.